ABSTRACT
With the acidification of paper-containing relics becoming increasingly serious, a convenient, effective and harmless method for deacidification has become an urgent necessity in the protection of paper relics. In this research, a novel method for reducing the acidity of paper by plasma technology is presented. which can be used simply at room temperature and atmospheric pressure. The pH of the paper rises to alkalescence rapidly after treatment and remains stable with no color change, with a slight accompanying increase in the mechanical properties of the paper.
Keywords Plasma treatment, Deacidification, Paper, Tensile strength
1. Introduction
In this paper, a cold plasma system has been used with a deacidification reagent consisting of saturated Ca(OH)2 solution to impregnate alkaline groups into the paper fibers and to modify the fifiber surfaces. Parallel investigations using traditional chemical deacidification treatments were carried out, using the same apparatus but without activating the plasma system, so that paper was only treated by Ca(OH)2, similar to the traditional aqueous deacidification method. Measurements of pH and tensile strength of the paper samples were also made in each case. Moreover, different kinds of colored paper were treated to measure the corresponding color change after plasma treatment. In addition, scanning electron microscopy was used to analyze the fiber surfaces of both treated and untreated samples, and the elemental content of the samples was measured by energy-dispersive X-ray spectroscopy[1].
2. Experimental
The experimental apparatus for plasma processing consists of an arc plasma gun, RF power supply (120-260W, 0-20 kHz), a conical flask to provide alkaline reagent, gas with valve and a flow meter, which is depicted in schematic form in Fig. 1. The saturated Ca(OH)2 solution firstly fills the plasma gun in vapor form, together with argon. The intensity of the plasma can be controlled by adjusting the flow rate of gas and the power of the generator. The arc plasma gun is connected to a computer numerical control electromotor in order to allow it to move on a designated route at a certain speed, so that every part of the paper placed in the attainable region of the plasma gun can be treated directly in the plasma zone for a certain period of time.
The experiments were carried out under the following working conditions: temperature 20-25℃, atmospheric pressure, output voltage 75V, arc power 100W. The gas used was argon with the rate of 4L/min, pH of alkaline agent was 12.48, the longitudinal speed of the plasma gun was 35 mm/s and the transverse distance was 2mm each time.

Fig. 1. Schematic of the specially designed plasma treatment apparatus.
2.3. Traditional parallel test method
A parallel test simulating a traditional aqueous deacidification method was made using the same experimental apparatus without activating the plasma system, so that the alkaline agent saturated Ca(OH)2 solution sprayed directly onto the paper samples from the inactive arc plasma gun. In this case, the samples were treated only by alkaline agent with all the other factors the same as for the plasma treatment process.
2.4. Accelerated aging procedure
The stability of the deacidification samples was investigated by moisture-heat-accelerated aging using an aging oven. Artificial aging was performed in the aging oven at a temperature of 80℃ and humidity of 65% for 72 hours, which corresponds to 25 years of natural aging[1].
2.5. Tensile tests
Tensile strength tests on paper sheets were performed on a computer controlled tensile testing machine with an extensometer gauge of 25mm and a test speed of 5mm/min. At least ten specimens, which were 100mm long and 15mm wide, were tested for each type of paper sheet in order to check for repeatability.
2.6. pH tests
The current surface method of evaluating the pH of paper using a flat electrode is known to be flawed, as it is really the pH of the solution that moistens the paper surface[13]. A more accurate but destructive method is cold extraction measurement[14]. For this method, paper samples are cut into pieces and dispersed in cold deionized water. Accordingly, 1g of the paper samples was added to 40ml of cold, deionized water for 1 hour and the pH of the water after the extraction time was deemed to be the pH of paper. For the present study, we used the non-destructive surface pH measurement technique to measure the pH of the paper relics, and the cold extraction method for ordinary samples.
2.7. Scanning electron microscope (SEM) and energy-dispersive X-ray spectroscopy (EDS) analysis
The surfaces of both untreated and plasma-treated samples were coated by evaporation with gold for 80 seconds before examination.Ascanning electronmicroscope (Hitachi-TM3000) was used to investigate the surface morphologies and determine the content of the specified elements at the same time.
3. Results and discussion
3.1. Measurement of properties before and after plasma deacidification
3.1.1. pH and tensile strength variation
After the deacidification treatment by plasma, both machine-made (Table 1) and hand-made (Table 2) paper samples experienced an increase in pH. Since there will be a long-term consumption of the alkaline compound,the pH achieved after treatment should be above neutral but not high enough to cause alkaline depolymerization.
Table 1 pH and tensile strength of machine-made paper before and after plasma treatment.

The optimal value was in the 8.0 range, as the experimental results showed. Furthermore, we can see that the tensile strength increased, especially for the hand-made paper, which was raised nearly 20%. This phenomenon is likely due to the effect of the plasma, since the high energy could change the structures of the fibers and accordingly improve the mechanical properties. Compared with the hand-made paper samples, machine-made paper contains large amounts of additives from the pulping process [15], which may influence the modification effects on the fiber surface.
Table 2 pH and tensile strength of hand-made paper before and after plasma treatment.
3.1.2. Tensile strength before and after aging
It is known that during the paper aging process, cellulose can depolymerize in the presence of acid, leading to acidification and fragility. The longer paper is kept over time, the more potential there is for it to be seriously affected by acidic substances. In this research, four typical kinds of Chinese paper samples were subjected to deacidification by plasma, and then aged artificially in the aging oven, imitating 25 years of aging under natural conditions, and then their tensile strength was measured.

Fig. 2. Results of tensile strength measurements before and after aging for the equivalent of 50 years for different plasma-treated and untreated samples.
The results of the aging study, presented in Fig. 2., show that after aging, the tensile strength of the blank samples was reduced sharply to nearly 70%, while the plasma-treated samples still maintained more than 85% of their tensile strength. If we compare the aging measurements, the treated samples still have much better tensile strength, which means that the plasma treatment improves the mechanical properties, together with deacidification.
3.1.3. Color changes
Molecular modifications in the cellulose polymers in paper caused by degradation reactions can be manifest in color changes. The evaluation of the color changes was measured by the CIE L*a*b* system[11]. The three parameters (L*, a*, b*) in the model represent the lightness (L*) of the color (the smallest value indicating black), a* its position between red and green (the smallest value indicating green) and b* its position between yellow and blue (the smallest value indicating blue) respectively. After the evaluation of parameters L*, a*, b*, the total color change △E*ab, before and after the plasma deacidification treatment, was determined, with the following equation:
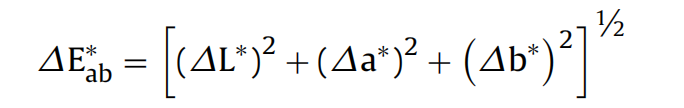
In Eq.(1), the smaller the value of △E*ab, the less difference there is between paper samples. In general, a△E*ab value of less than 1.5 is deemed to be undetectable by the human eye.
Table 3 Color change of various hand-made paper samples.

In the present study, three kinds of color changes were measured for every sample. The color change ofthe same treated sample before and after deacidification by plasma is given by △E1. △E2 represents the color change between treated and untreated samples before aging, while △E3 is for samples after aging. The color changes for five different types of hand-made paper samples are presented in Table 3. These results indicate that all the samples show little color change between treated and untreated samples, proving that plasma treatment does not change the color of the paper[16]. The artificial aging revealed a relatively large difference between treated and untreated samples according to △E3 compared with the variation between aged and un-aged samples after plasma treated in △E2. Since the yellowing and darkening of cellulose is related to the acid hydrolysis and oxidation of the polymer chains, and after aging the treated samples showed a similar color, it is evident that the plasma treatment can decrease the speed of cellulose degradation to some extent.
Different kinds of pigments and colored papers were treated and the corresponding △E*ab values before and after deacidification were measured (Table 4).
Table 4 Color change of different colored papers and pigments.
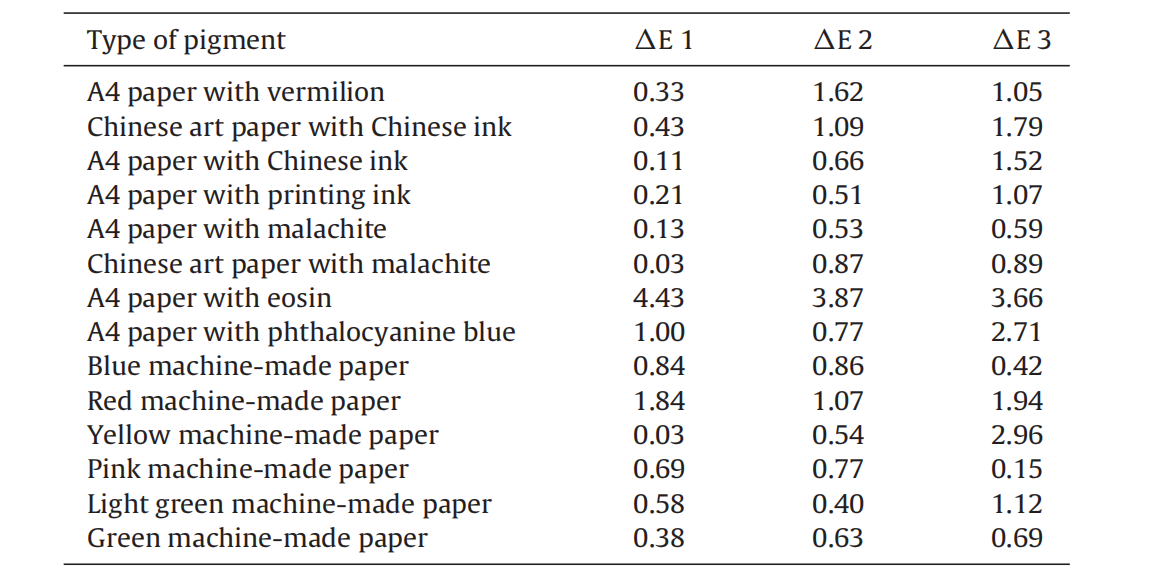
For most of the pigments and colored papers, the color changes were small and random, so that we can consider that no real color change takes place after deacidification treatment, with the exception of red pigment. Since the carbonyl groups in the cellulose and pigment are chromophores [17], the observation of little color change means that plasma treatment does not change the structure of the polymer chains or accelerate the hydrolysis reaction. For the eosin, whose main component is tetrabromoflfluorescein, the phenolic groups would easily be oxidized during the aging process, which leads to the large observed changes of color.
3.2. Plasma vs. chemical treatment
Most tradition deacidification methods using chemical reagents such as Ca(OH)2 directly show a significant effect immediately up on application. In the present study, we use both chemical and plasma treatments for deacidification in order to compare the methods and observe any differences between them (Table 5).
Table 5 pH comparison between chemical and plasma treatments of paper samples.
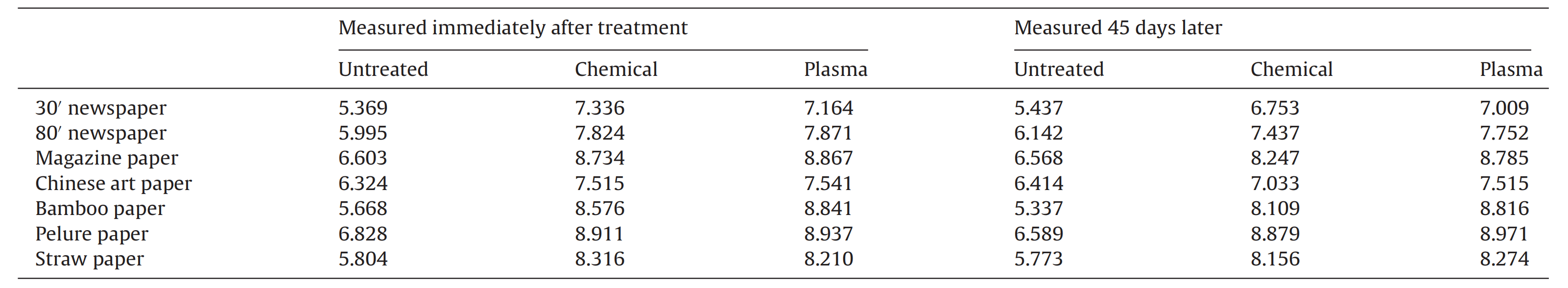
The pH of all types of samples increased substantially if measured immediately after deacidification with both chemical and plasma treatments. However, we kept the treated samples in a closed environment for one and half a months and measured the pH again. After 45 days, we obtained an interesting result, where by the pH of the sample, which was treated by the chemical method, showed an obvious decrease, while those treated by the plasma method maintained a pH similar to that of the previous 45 days.
This result illustrates that the effect of deacidifification lasts longer when using the plasma method compared to the traditional chemical deacidification treatment.
 Fig. 3. pH variation of paper treated by different deacidification methods over 90 days' post-treatment time.
Fig. 3. pH variation of paper treated by different deacidification methods over 90 days' post-treatment time.
In order to study the process of pH change, a large piece of 1940's newspaper was cut into equal halves, with one half treated by the chemical method and the other treated by plasma. Both of the paper samples were kept in a closed environment and the pH was measured every few days by the cold extraction method to obtain the accurate pH. From the results shown in Fig. 3, the pH of the chemically treated sample dropped gradually, while that of the sample treated by plasma remained unchanged. After 45 days, the pH trend of both samples remained stable, with the alkalinity of the plasma-treated sample being much stronger, while the pH of the other sample was nearly the same as that at the beginning of the study.
3.3. Elemental analysis and surface morphology
Energy-dispersive X-ray spectroscopy (EDS) was used to analyze the elemental composition of the fibers of the paper surfaces, with the results given in Table 6. We found that the content of both C and O changed with increasing the content of Ca2+ after deacidification by both methods. However, for the composition ratio of O/C, the plasma-treated sample showed a notable difference. While both the untreated and chemically treated samples had the same ratio of 1.17, the ratio of O/C rose to 1.23 for the plasma-treated sample, which means that the chemical structure of the fiber surface was influenced by the plasma treatment, with some new oxygen groups created[7].
Table 6 Elemental composition of fibers obtained from energy-dispersive X-ray spectroscopy (EDS).

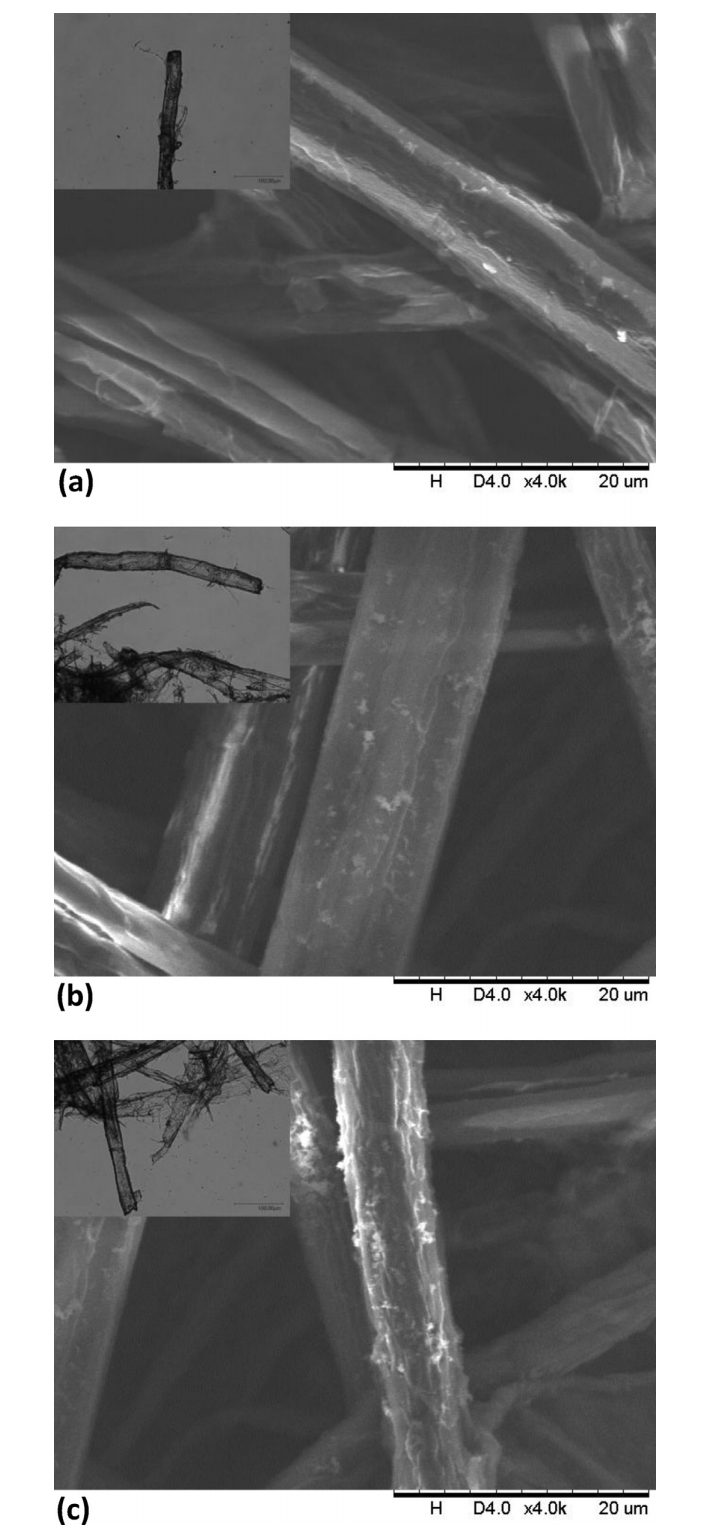
Fig. 4. shows the optical microscopy results and scanning electron micrographs of the fiber surfaces of untreated, chemically treated and plasma treated paper samples. The main structure of the fibers treated by plasma has changed very little compared with the untreated sample under the optical microscope. However, it is clearly seen that the surface of the fibers became rough after treatment by plasma while the surface of the other two samples remained smooth. There were many fiber branches, similar to the devillicate formed during the paper pulping process, which means that the high-energy plasma does not damage the polymer chains; instead, it modifies the surface. The surface of the fibers was activated and split into many tiny fibers, which increases the specific surface area and exposes more hydroxyl groups, allowing them to form more hydrogen bonds with neighboring fibers. Meanwhile, the plasma process may also produce new oxygenated species as indicated by the EDS result, which can also form more hydrogen bonds. Thus,the number of connection points between fibers would increase, thereby enhancing the bonding forces between fibers in the paper, ultimately resulting in improved mechanical behavior in the paper in macroscopic terms.
3.4. Plasma technology applied to paper relics
An acidified page of an authentic book from the Ming dynasty(about 1600 A.D.) was chosen as the test subject for this procedure, and pH measurements the CIE L*a*b* system were used to determine the effificiency of plasma deacidification on this example of a paper relic.

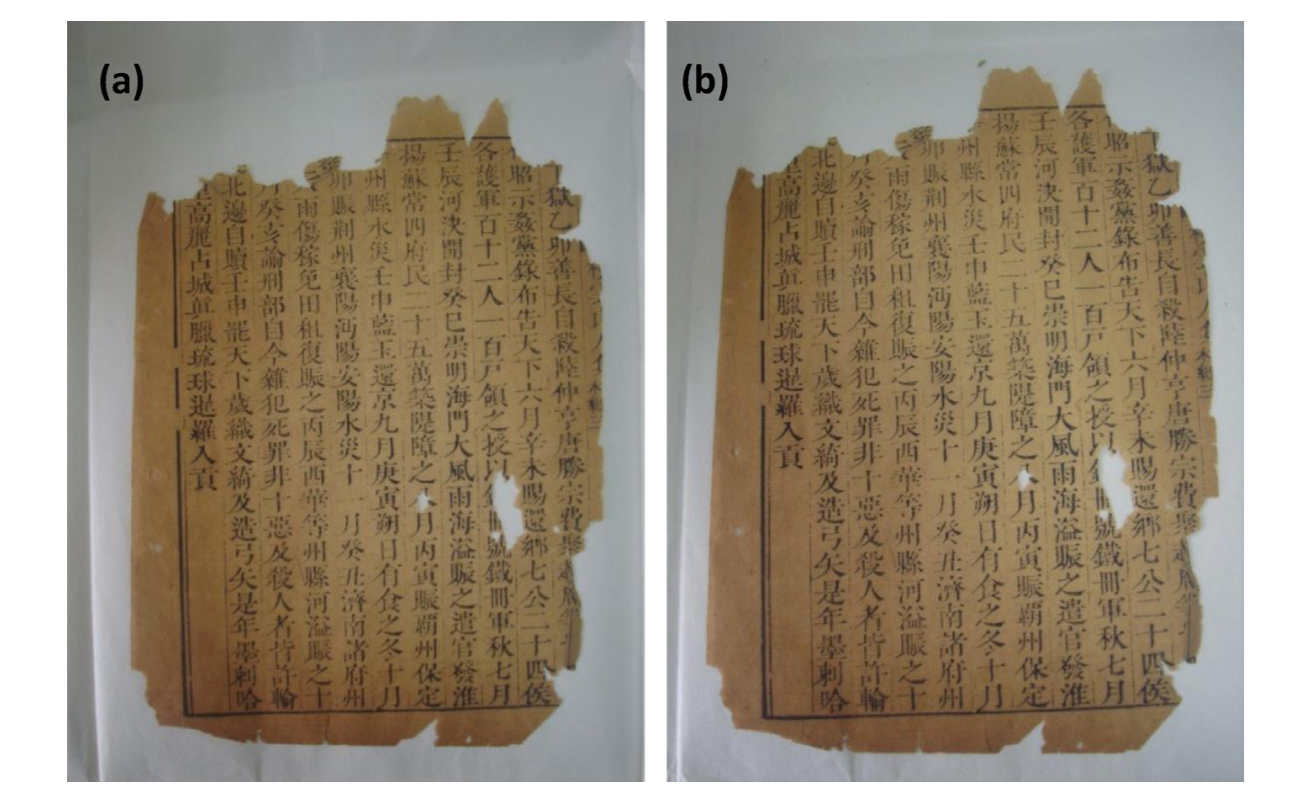
After application of the plasma deacidification technique, the pH of the page rose from 4.478 to 7.737 (Fig.5.), demonstrating that the paper changed to alkalescency after treatment, without any other apparent changes to the paper (Fig. 6). The L*a*b* chromatic aberration analysis for the same point before and after treatment presented in Table 7, shows that the color change for the ancient page was around 1.0, which confirms that the plasma deacidification method applied to paper relics only lowers the degree of acidification, and does not influence the appearance of the paper or the pigment and writing on it.
Table 7 Color change for untreated and treated paper relics.
4. Conclusions
This report presents a novel deacidification method for paper relics using atmospheric plasma technology with advantages over the present wet-chemical-based deacidification methods, including shortening the deacidification duration from several hours to just a few minutes, avoiding paper crinkle, color change and visual appearance altering caused by direct contact of paper with solution-stated deacidification reagents, and prolonging the maintaining effect for paper deacidification. The proposed plasma technology could strengthen the paper since part of shallow surface fibers might be activated and split into tiny fibers to form more hydrogen bonds with neighboring fibers, while the original appearance of paper could be preserved due to the short plasma processing period that avoids the degradation of cellulose.
Acknowledgements
This work was supported by grants from the Foundation of State Administration of Cultural Heritage of China (No. 20110105), and from the Foundation of Cultural Heritage Conservation Science and Technology Project of Zhejiang in 2012 (Application of plasma deacidification key technology in deacidification of neoteric and modern paper relics).
Written By Qinglian Li a, Sancai Xi b, Xiwen Zhang a,b,∗
a Department of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, China
b Research Institute of Cultural Heritage, Zhejiang University, Hangzhou 310027, China
References
[1] M.C. Area, Paper aging and degradation: recent fifindings and research methods, Bioresources 6 (2011) 5307–5337.
[2] H.A. Carter, The chemistry of paper preservation: part 1. The aging of paper and conservation techniques, J. Chem. Educ. 73 (1996) 417–420.
[3] J.W. Baty, Deacidification for the conservation and preservation of paper-based works: a review, Bioresources 5 (2010) 1955–2023.
[4] M.S. Rakotonirainy, A. Dupont, B. Lavédrine, S. Ipert, H. Cheradame, Mass deacidification of papers and books: V. Fungistatic properties of papers treated with aminoalkylalkoxysilanes, J. Cult. Herit. 9 (2008) 54–59.
[5] H. Cheradame, Mass deacidification of paper and books. I: study of the limitations of the gas phase processes, Restaurator 24 (2003) 227–239.
[6] Y. Wang, Y. Fang, W. Tan, C. Liu, Preservation of aged paper using borax in alcohols and the supercritical carbon dioxide system, J. Cult. Herit. 14 (2013) 16–22.
[7] X. Yuan, K. Jayaraman, D. Bhattacharyya, Effects of plasma treatmentin enhancing the performance of woodfibre-polypropylene composites, Compos. Part A Appl. Sci. Manuf. 35 (2004) 1363–1374.
[8] H. Bansa, Aqueous deacidification-with calcium or with magnesium? Restaurator 19 (1998) 1–40.
[9] E. Stefanis, Deacidification of documents containing iron gall ink with dispersions of Ca(OH)2 and Mg(OH)2 nanoparticles, Restaurator 31 (2010) 19.
[10] L. Botti, The effect of sodium and calcium ions in the deacidification of paper: a chemo-physical study using thermal analysis, Restaurator 27 (2006) 9–23.
[11] S. Sequeira, C. Casanova, E.J. Cabrita, Deacidification of paper using dispersions of Ca(OH)2 nanoparticles in isopropanol. Study of efficiency, J. Cult. Herit. 7 (2006) 264–272.
[12] J. Bogaard, P.M. Whitmore, Effects of dilute calcium washing treatments on paper, J. Am. Inst. Conserv. 40 (2001) 105–123.
[13] H. Clark, J. Gibbs, A. Jarjis, An investigation into the deacidification of paper by ethoxymagnesium ethylcarbonate, J. Mater Chem. 8 (1998) 2685–2690.
[14] TAPPI T 509 om-02 – Hydrogen ion concentration (pH) of paper extracts (cold extraction method).
[15] M.A. Hubbe, Handmade paper: a review of its history, craft, and science, Bioresources 4 (2009) 1736–1792.
[16] E.G. Ioanid, A. Ioanid, D.E. Rusu, C. Popescu, I. Stoica, Surface changes upon highfrequency plasma treatment of heritage photographs, J. Cult. Herit. 12 (2011) 399–407.
[17] V. Bukovsky, Yellowing of newspaper after deacidification with methyl magnesium carbonate, Restaurator 18 (1997) 25–38.



